Corrective and Preventive Action System for ISO & IATF Compliance
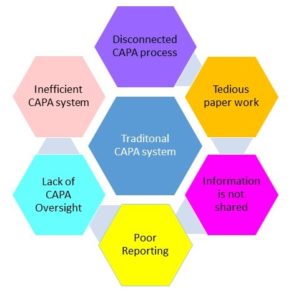
Challenges:
Inefficient and difficult to maintain:
Paper-based and hybrid systems for Corrective Action and Preventive Action are inexpensive initially. In the long term, however, these systems are inefficient, requiring tremendous man-hours in terms of routing CAPA tasks and other documentation, obtaining approval and signatures, and manual search and retrieval of documents during inspections and audits.
No Integration and information Sharing:
A CAPA may be triggered by Form 483 findings, ISO quality audits, customer complaints, or some other source. With manual and hybrid systems, these sources are not connected, making data collection slow and incomplete. Without connectivity, critical information may fall through the cracks, and the root cause investigation is likely to be unreliable.
Lack of CAPA Oversight:
Poor implementation of CAPA systems a top reason for issuance of a Form 483) may stem from the lack of ability to track and monitor open CAPAs and proactively improve the CAPA process
Solutions:
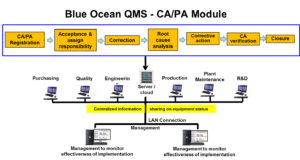
Blue Ocean CAPA Systems are designed by industry practitioners and experienced quality consultant for automating the Corrective and Preventive Action (CAPA) process in any organization.
An automated CAPA system reduces audit time and findings, and decreases risk of product recalls. It improves product quality and safety, increases customer satisfaction, and ensures FDA and ISO compliance.
Blue Ocean Corrective Action software is a robust, easy-to-use system designed to effectively manage the corrective action / preventive action process and integrate it with other quality processes critical to regulatory compliance, such as change control, audit, and customer complaints.
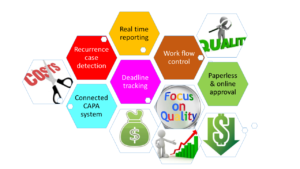
Key benefits and features:
Real time Reporting:
Blue Ocean Corrective Action software tracks quality incidents that can escalate into a CAPA, such as customer complaints, audit findings, etc. The system provides advanced analytics and reporting capability, including customizable reports and online charting. Through the reports, managers get a real-time view of the CAPA process and can be more proactive about improving their quality system.
Centralized information management:
The Blue Ocean Corrective Action system automates routing, notification, delivery, escalation and approval of CAPAs and all related documentation. It automates management of entire CAPA process, from initiation to investigation and all the way through closure. Provides a secure, centralized, and Web-based repository for all CAPA documents.